Protecting the Visual Pathways During Optic Nerve Surgery Using Intraoperative Visual Evoked Potentials (VEP)
December 17, 2019
Faisal R. Jahangiri, M.D., CNIM, D.ABNM, FASNM, FASET1, Sidra Ilyas, MD2, Rabehah Asdi3, Esther Escobedo, CNIM1, Michael Seals, MD1
1Axis Neuromonitoring LLC, Richardson, TX, USA; 2Dept. of Anatomy, School of Medicine, University of California, San Francisco, CA; 3Student, School of Behavioral and Brain Sciences, The University of Texas, Richardson, USA.
Objective:
This case report illustrates the benefit of utilizing Intraoperative Neurophysiological Monitoring (IONM) during the resection of an optic nerve lesion. The IONM utilized Electroretinogram (ERG), Visual Evoked Potentials (VEP) and Electroencephalography (EEG).
Case Report:
A 47-year-old female presented with left planum sphenoidale and tuberculum meningioma. The patient had a history of hypothyroidism and decreased vision in the right eye. An MRI showed tumor attached to the left optic nerve and posteriorly displacing optic nerve and chiasm to the right. A craniotomy with a total resection was planned. Total intravenous anesthesia (TIVA) was used with Propofol infusion. After induction and patient positioning, eyes were closed using transparent tape. LED Goggles were placed and secured on eyes bilaterally for performing visual evoked potentials (VEP). Subdermal needle electrodes were placed on occipital lobes for VEP recordings. Subdermal needle electrodes were also placed scalp for EEG recordings.
At the baseline the VEP responses were absent. The anesthesiologist was consulted and was requested to stop the inhalational agent (sevoflurane) and switch to total intravenous anesthesia (TIVA). After switching to TIVA, Electroretinography (ERG) responses were present bilaterally. Baseline VEP and EEG recordings were obtained with good left VEP and absent right VEP responses. During tumor resection there was sudden decrease in left VEP responses. Retractors were removed immediately and the responses came back to baseline within few minutes. Tumor resection was completed without any event.
Discussion:
Intraoperative use of visual evoked potentials (VEP) during the surgical procedures involving the optic nerve can be highly beneficial to the patient and as well as the surgeon. Utilizing the ERG and VEP during manipulation and tumor resection helps in identifying any pressure or stretch on the optic nerve. However, it is important to remember that intraoperative visual testing is only reliable for surgeries involving areas around optic chiasm and optic nerves. Real-Time feedback to the surgeon about the visual pathways can minimize any post-operative visual deficits.
Conclusion:
In this patient, the utilized visual evoked potentials (VEP) were effectively used for the prevention of any loss of vision intra-operatively. The neurophysiological monitoring utilizing ERG and visual evoked potentials (VEP) were helpful in preventing any further loss of vision and directing the surgeon intra-operatively. VEP can be abnormal in brain injuries, optic neuritis, and neuropathy, tumors compressing the optic nerve, retrobulbar neuritis surgery. Postoperative visual loss is a devastating complication of brain surgery. During surgeries that put the visual pathway at risk of injury, continuous monitoring of the visual function is desirable. However, the intraoperative monitoring of the visual evoked potential (VEP) is not yet widely used.
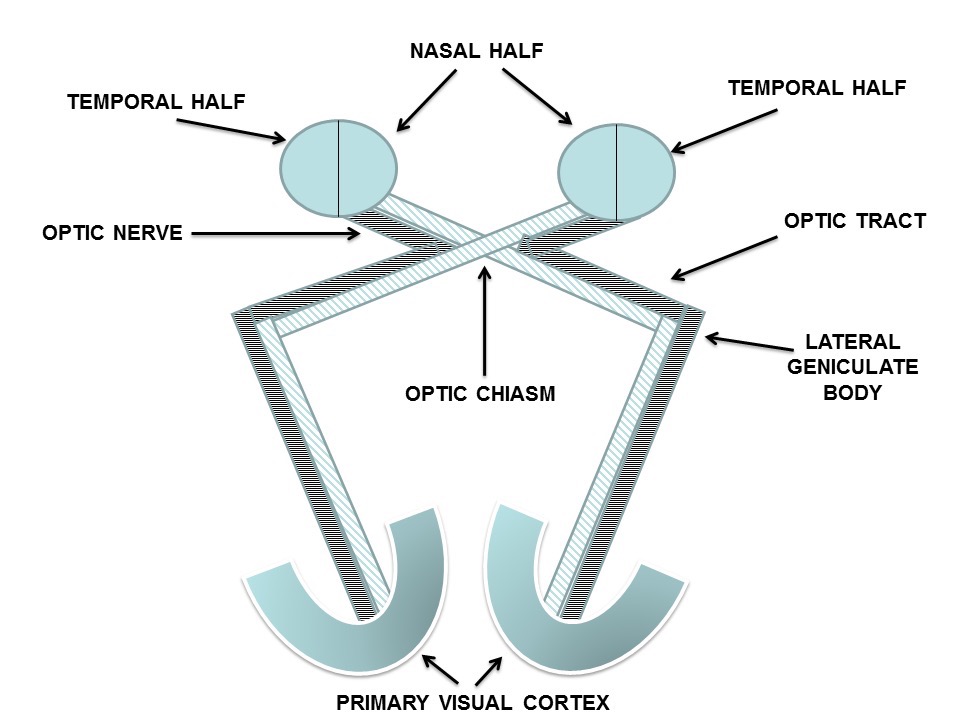
Figure 1: Schematic diagram of the visual pathways (reprinted with permission from Faisal R. Jahangiri (2012), Surgical Neurophysiology: A Reference Guide to Intraoperative Neurophysiological Monitoring (IONM), CreateSpace, Columbia, SC.
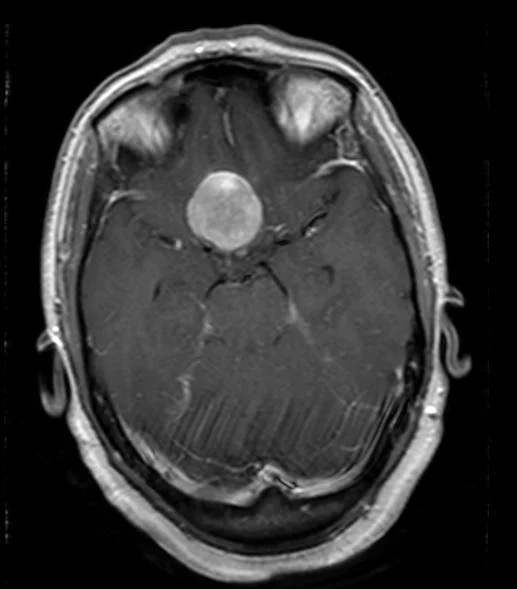
Figure 2: Pre-op MRI Axial View: A large 2x2.5x3 cm T1 isointense lesion homogenously enhancing with contrast seen over the planum sphenoidale extending posteriorly to the suprasellar cistern causing mass effect on the adjacent posterior frontal gyri and posteriorly displacing the optic nerves and chiasm and stalk of the pituitary gland.
Figure 3: Pre-op MRI Sagittal View:
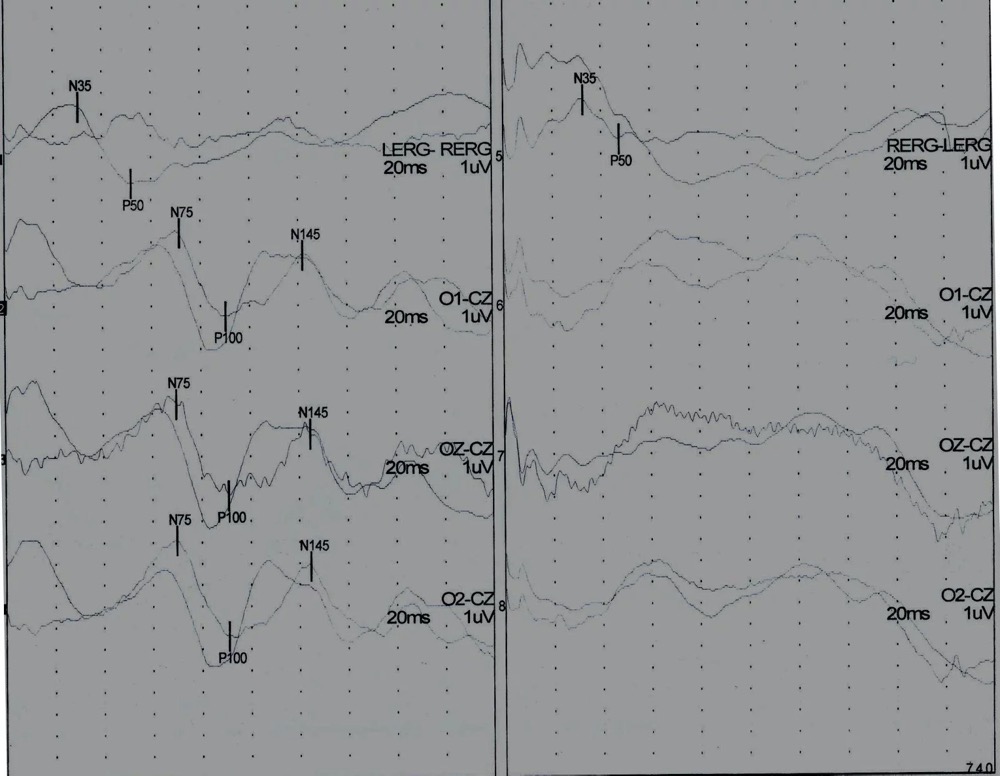
Figure 4: Baseline Electroretinogram (ERG), Visual Evoked Potentials (VEP) responses showing present ERG and VEP responses to left eye stimulation (Left), and present ERG and absent VEP responses to right eye stimulation (Right).
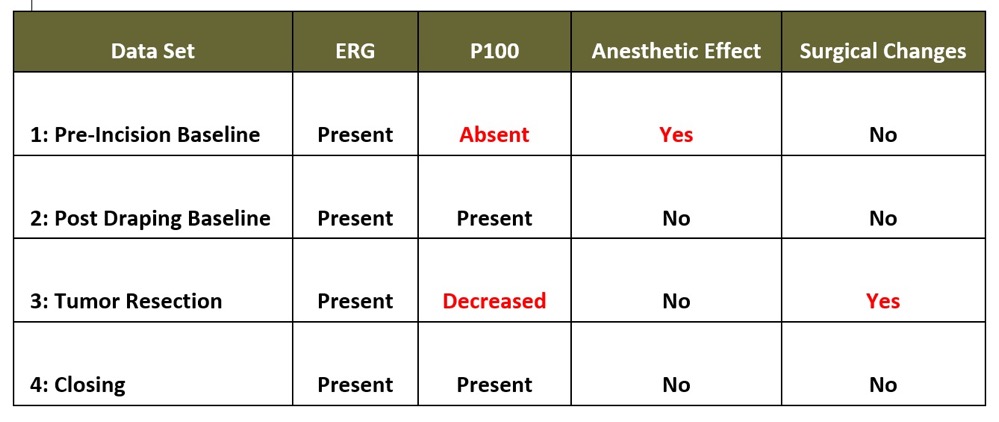
Table 1. Intraoperative Electroretinogram (ERG), Visual Evoked Potentials (VEP) Data
Figure 5: Post-op MRI: Surgical Cavity is demonstrated along the posterior aspect of the gyrus with no residual disease identified. Postoperatively the compression on the chiasm has been significantly resolved secondary to the complete resection of the meningioma.
The intraoperative neuromonitoring was performed by a CNIM certified technologist and a D.ABNM board-certified neurophysiologist. A board-certified neurologist with a specialty in IONM was also present online for remote monitoring during the entire surgical procedure.
Results:
In this patient, the intraoperative neurophysiological monitoring (IONM) helped in guiding the surgeon during the high-risk procedure. Due to a sudden decrease in left VEP responses surgeon was alerted and this resulted in no postoperative neurological deficit. Tumor attached to left optic nerve was resected without any loss of vision intraoperatively.
Post-operative:
The patient noticed an improvement in her right eye four days post-operatively. At one-month post-operative follow-up she continued to feel improvement.
References
- ABRET Neurodiagnostic Credentialing and Accreditation. (2019, Sep 9). ABRET. Retrieved Sep 9, 2019, from ABRET: https://www.abret.org/candidates/credentials/cnim/
- ABNM: American Board of Neurophysiologic Monitoring. (2019, Sep 9). ABNM: American Board of Neurophysiologic Monitoring. Retrieved Sep 9, 2019, from ABNM: American Board of Neurophysiologic Monitoring: http://www.abnm.info/
- Gertsch JH, Moreira JJ, Lee GR, Hastings JD, Ritzl E, Eccher MA, Cohen BA, Shils JL, McCaffrey MT, Balzer GK, Balzer JR, Boucharel W, Guo L, Hanson LL, Hemmer LB, Jahangiri FR, Mendez Vigil JA, Vogel RW, Wierzbowski LR, Wilent WB, Zuccaro JS, Yingling CD; membership of the ASNM. (2019). Practice guidelines for the supervising professional: intraoperative neurophysiological monitoring. J Clin Monit Comput, 33(2), 175-183. doi:10.1007/s10877-018-0201-9
- Jahangiri, FR. Surgical Neurophysiology: An Introduction to Intraoperative Neurophysiological Monitoring (IONM). Second Edition. 193-95, April 2012.
- Boomer JA, Siatkowski RM (2003). "Optic neuritis in adults and children". Seminars in ophthalmology 18 (4): 174–80.
- Lucchinetti, C. F.; L. Kiers, A. O'Duffy, M. R. Gomez, S. Cross, J. A. Leavitt, P. O'Brien, and M. Rodriguez (November 1997). "Risk factors for developing multiple sclerosis after childhood optic neuritis". Neurology 59 (5): 1413–1418.
- Cervetto L, Demontis GC, Gargini C (February 2007). "Cellular mechanisms underlying the pharmacological induction of phosphenes". Br. J. Pharmacol. 150 (4): 383–90.
- Ezzat S, Asa SL, Couldwell WT, Barr CE, Dodge WE, Vance ML, McCutcheon IE. (August 2004). "The prevalence of pituitary adenomas: a systematic review". Cancer 101 (3): 613–9.
- Asa SL (August 2008). "Practical pituitary pathology: what does the pathologist need to know?". Arch. Pathol. Lab. Med. 132 (8): 1231–40. Retrieved 2011-12-03.
- Halliday AM, editor. Evoked potentials in clinical testing, 2nd ed. London: Churchill Livingstone, 1993.
- Cedzich C., Schramm J, Fahlbusch R. Are flash-evoked visual potentials useful for intraoperative monitoring of visual pathway function? Neurosurgery 21: 709-715, 1987.
- McDonald, W. I., Compston, A., Edan, G., et al (2001), Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis.
- Annals of Neurology, 50:121–127. doi:10.1002/ana.1032
- Dale RC, DeSousa C, Chong WK, et al. Acute disseminated encephalomyelitis and multiple sclerosis in children. A follow-up study to compare clinical and investigative findings on disease presentation. Brain2000;123:2407–2422.
- Thirumala PD, Habeych ME, Crammond DJ, Balzer JR. Neurophysiologic Intraoperative Monitoring of Olfactory and Optic Nerves. J Clin Neurophysiol. 2011 Dec 5.
- Genetic Reactivation of Cone Photoreceptors Restores Visual Responses in Retinitis pigmentosa. Volker B, Jens D, David B et al. Science 1190897Published online 24 June 2010 [DOI:10.1126/science.1190897]



