Intraoperative Neurophysiological Monitoring for Brainstem Tumor Surgeries
August 27, 2020
Intraoperative Neurophysiological Monitoring for Brainstem Tumor Surgeries
Katharine Pautler1,2, Elizabeth Ekvall1,2, Meredith Tucker2,
Angela Goodlin1,2, Afia Islam2, Faisal R. Jahangiri1,2
1Axis Neuromonitoring LLC, Richardson, Texas, USA.
2School of Brain and Behavior Sciences, The University of Texas at Dallas, Richardson, Texas;
Intraoperative Neurophysiological Monitoring (IONM) is routinely utilized to detect and prevent injuries to the nervous system during surgeries. IONM can be used to map and monitor cranial nerves that may be displaced due to large tumors or at risk of being damaged during tumor resection. Surgical procedures involving the removal of brainstem tumors may risks damage to the Facial nerve (cranial nerve VII) and Vestibulocochlear Nerve (Auditory nerve / cranial nerve VIII). Any injury to the Facial nerve can lead to facial muscle paralysis, loss of taste sensation, and tear production. Lack of tear production can result in damage to the cornea that may lead to loss of vision. Injury to the auditory nerve due to stretch, thermal, or ischemia (loss of blood supply) has a high risk of postoperative hearing deficits or hearing loss. Intraoperative neuromonitoring can be used to mitigate deficits when resecting various brainstem tumors. During brainstem tumor resections, a multimodality neuromonitoring approach including Somatosensory Evoked Potentials (SSEPs), Motor Evoked Potentials (MEPs), Brainstem Auditory Evoked Potentials (BAEP), Cranial Nerve Electromyography (CN-EMG) should be used. IONM is crucial for minimizing and preventing damage to the cranial nerves, damage to corticospinal tracts, and ischemia. The likelihood of injury, the severity of the damage, and permanency of loss can be minimized with IONM by alerting the surgeon about the changes in the recorded data that have occurred before the damage is irreversible. Large tumors, especially those larger than 2.5 cm, may shift anatomy in the brainstem and will require monitoring of the lower cranial nerves from IX to XII, but a multimodality IONM can be used for mapping and minimizing any postoperative neurological deficit.
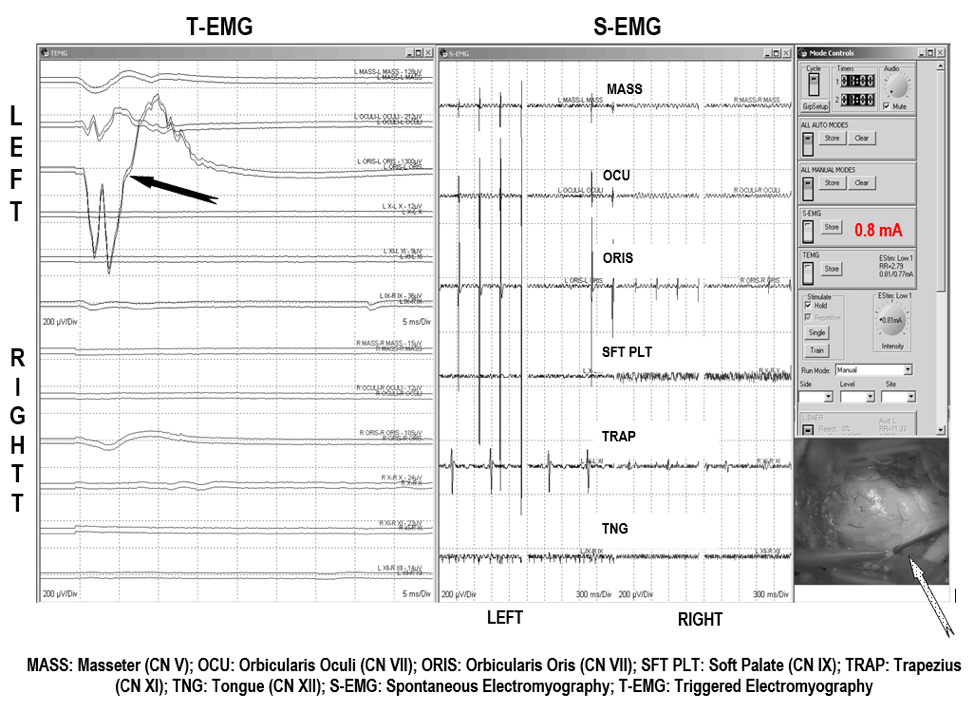
Figure 1. Facial Nerve Electromyography (EMG). EMG recordings are showing Triggered EMG (t-EMG) views on a response from Facial nerve (orbicularis oculi and orbicularis oris muscles) after stimulating (black arrow). This response localized and confirmed the involvement of the facial nerve (CN VII) within the tumor. Lower right: Tumor in the fourth ventricle (white dotted arrow).in the fourth ventricle (white dotted arrow). (Jahangiri et al., 2012).
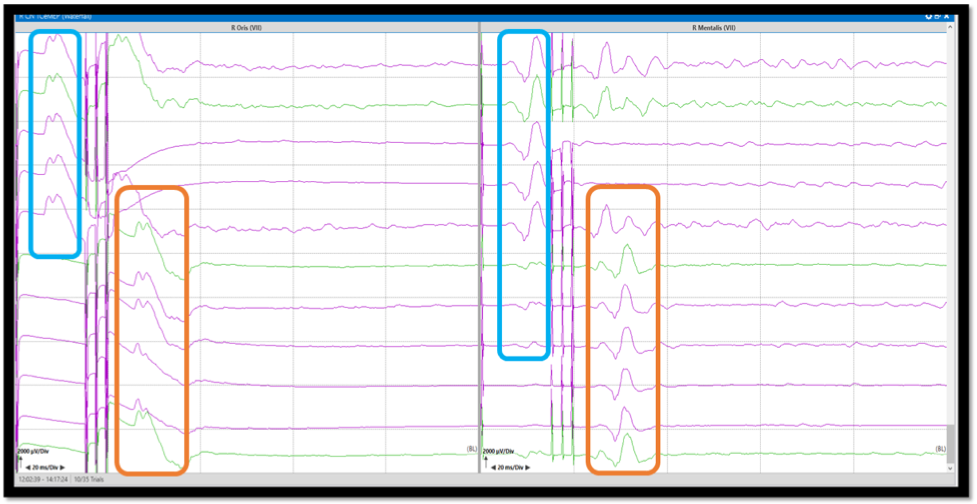
Figure 2. Corticobulbar Motor Evoked Potentials (Co-MEP). Co-MEP recordings from orbicularis oris and mentalis muscles with double train stimulation (first pulse single, second train three pulses). The first single pulse responses are recorded due to direct surface activation of the facial muscles (blue box). The second train pulses activate the facial muscles through the corticobulbar pathways (orange box). The stimulation should be decreased or adjusted until the response from the single pulse disappears.
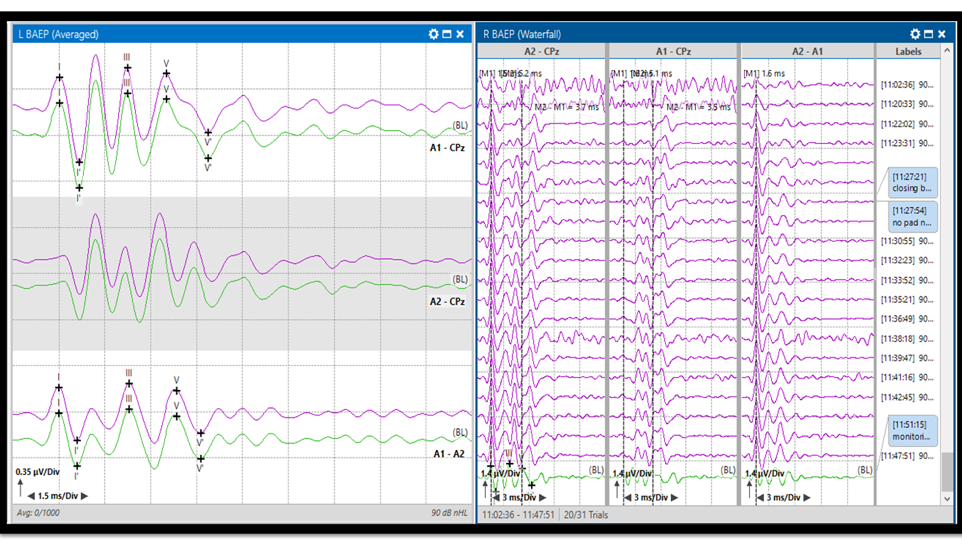
Figure 3. Brainstem Auditory Evoked Potentials (BAEP). BAEP response in average view (left) with baselines (green) and last average responses (purple). BAEP stack view (right). The responses are recorded from the distal auditory nerve, proximal auditory nerve, and the brainstem (Waves I, II, III, IV, and V).
A brainstem tumor is an abnormal growth of tissue within the brainstem, from the rostral midbrain to the caudal medulla. There are four main types of brainstem tumors. The majority of the present research focuses on; gliomas in children and schwannomas in adults. Other major types of brainstem tumors are brainstem neuromas and meningiomas. Brainstem gliomas are the most common type of brainstem tumors, making up 78% of all malignant brain tumors (AANS, 2020), with a significant portion of gliomas tumors being diagnosed in childhood (Hu et al., 2016). There are two subtypes of brainstem gliomas; tectal gliomas and pontine gliomas. Tectal gliomas are the more common and less severe of the two (Hu et al. 2016). The second most common type of brainstem tumor is Schwannoma and is a tumor of the Schwann cells. There are four subtypes of brainstem schwannomas, each ranging in severity (Pinna et al. 2012). However, the most common type of Schwannoma is an acoustic neuroma (AANS, 2020).
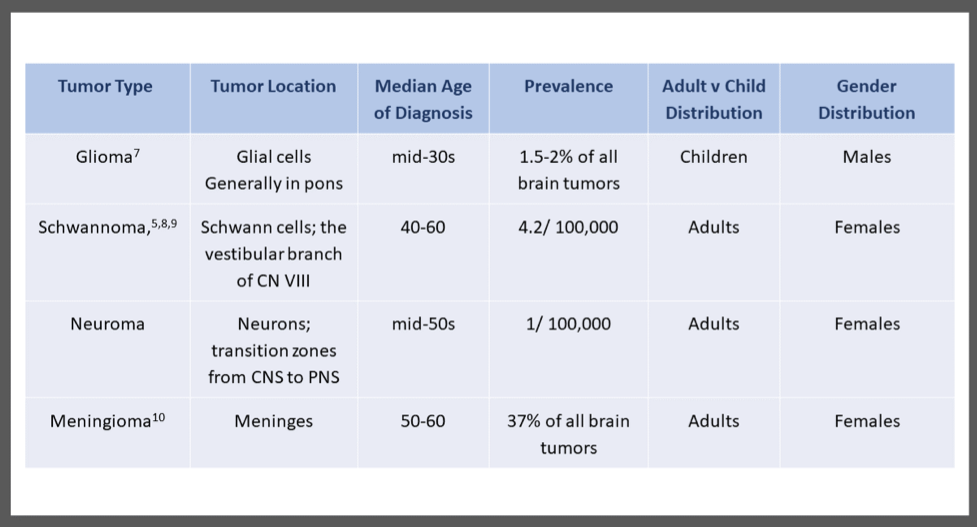
Table 1. The most common type of brainstem tumor in adults.
Schwannomas almost always involve the cranial nerves and stem from the vestibular branch of the eighth cranial nerve, the vestibulocochlear nerve (Xinrui et al., 2017). The third m2ajor type of brainstem tumors is a brainstem neuroma, which is a tumor of neurons. These tumors typically form at the junction between CNS and PNS within the supratentorial region (Molnar 2019). Meningioma is a tumor of the meninges and generally has a reasonably good prognosis. However, they are challenging to operate on given the nature of the growth of the tumors (Bilgin et. a., 2019). They are the most common type of brainstem tumor in adults (AANS, 2020). (Table
References
- Types of Brain Tumors. (2020). American Association of Neurological Surgeons. Retrieved February 12, 2020, from https://www.aans.org/en/Patients/Neurosurgical-Conditions-and-Treatments/Brain-Tumors
- Hu J, Western S, Kesari S. Brainstem Glioma in Adults. Front Oncol. 2016;6:180. Published 2016 Aug 9. doi:10.3389/fonc.2016.00180
- Pinna, M. H., Bento, R. F., & Neto, R. V. (2012). Vestibular Schwannoma: 825 cases from a 25-year experience. International archives of otorhinolaryngology, 16(4), 466–475. https://doi.org/10.7162/S1809-97772012000400007
- Yang DX, Jing Y, Xu ZM, et al. Primary Glioblastoma of Cerebellopontine Angle in Adult Mimicking Acoustic Neuroma. World Neurosurg. 2019;122:48-52. doi:10.1016/j.wneu.2018.10.073
- Molnar, H. (2019). Acoustic Neuroma: Overview. Retrieved from https://www.hopkinsmedicine.org/otolaryngology/specialty_areas/otology/conditions/acoustic-neuroma/
- Bilgin, E., Çavus, G., Açik, V., Arslan, A., Olguner, S. K., Istemen, I., Gezercan, Y., & Ökten, A. I. (2019). Our surgical experience in foramen magnum meningiomas: Clinical series of 11 cases. The Pan African medical journal, 34, 5. https://doi.org/10.11604/pamj.2019.34.5.17536
- Kerezoudis P, Goyal A, Lu VM, et al. The role of radiation and chemotherapy in adult patients with high-grade brainstem gliomas: results from the National Cancer Database. J Neurooncol. 2020;146(2):303-310. doi:10.1007/s11060-019-03374-x
- Xinrui L, Sato Y, Dan M, Kuroda H, Kumabe T. Total Resection of Brainstem Extension of Tentorial Schwannoma Using an Occipital Transtentorial Approach. World Neurosurg. 2017;98:879.e13-879.e16. doi:10.1016/j.wneu.2016.11.049
- Marinelli JP, Lohse CM, Carlson ML. Incidence of Vestibular Schwannoma over the Past Half-Century: A Population-Based Study of Olmsted County, Minnesota. Otolaryngol Head Neck Surg. 2018;159(4):717-723. doi:10.1177/0194599818770629
- Jiang XB, Ke C, Han ZA, et al. Intraparenchymal papillary meningioma of brainstem: case report and literature review. World J Surg Oncol. 2012;10:10. Published 2012 Jan 12. doi:10.1186/1477-7819-10-10
- Jahangiri, F. (2012). Surgical Neurophysiology: A reference guide to intraoperative neurophysiological monitoring (IONM). Createspace, SC.
- Jahangiri FR, Minhas M, Jane J Jr. Preventing lower cranial nerve injuries during fourth ventricle tumor resection by utilizing intraoperative neurophysiological monitoring. Neurodiagn J. 2012;52(4):320-332.
- Kim, K., Cho, C., Bang, M.-S., Shin, H.-I., Phi, J.-H., & Kim, S.-K. (2018). Intraoperative Neurophysiological Monitoring : A Review of Techniques Used for Brain Tumor Surgery in Children. Journal of Korean Neurosurgical Society, 61(3), 363–375. doi: 10.3340/jkns.2018.0078
- American Clinical Neurophysiology Society. (2009). Recommended Standard for Intraoperative Monitoring of Auditory Evoked Potentials. Retrieved from https://www.acns.org/pdf/guidelines
- Macdonald DB, Skinner S, Shils J, Yingling C; American Society of Neurophysiological Monitoring. Intraoperative motor evoked potential monitoring - a position statement by the American Society of Neurophysiological Monitoring. Clin Neurophysiol. 2013;124(12):2291-2316. doi:10.1016/j.clinph.2013.07.025



